
The H2S molecular geometry is a diagram that illustrates the number of valence electrons and bond electron pairs in the H2S molecule in a specific geometric manner. The first step is to sketch the molecular geometry of the H2S molecule, to calculate the lone pairs of the electron in the central sulfur atom the second step is to calculate the H2S hybridization, and the third step is to give perfect notation for the H2S molecular geometry. Key Points To Consider When drawing The H2S Molecular GeometryĪ three-step approach for drawing the H2S molecular can be used. What is the molecular notation for H2S molecule?.Molecular Geometry Notation for H2S Molecule :.Calculate the number of molecular hybridizations of the H2S molecule.Calculating lone pair of electrons on hydrogen in the H2S geometry:.Calculating lone pairs of electrons on sulfur in the H2S geometry:.
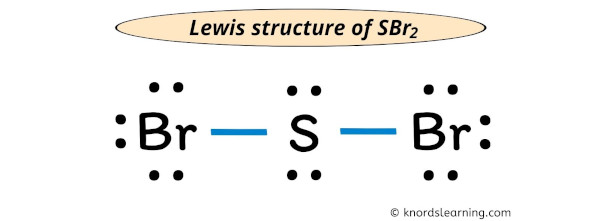
Sbr2o electron domain geometry how to#
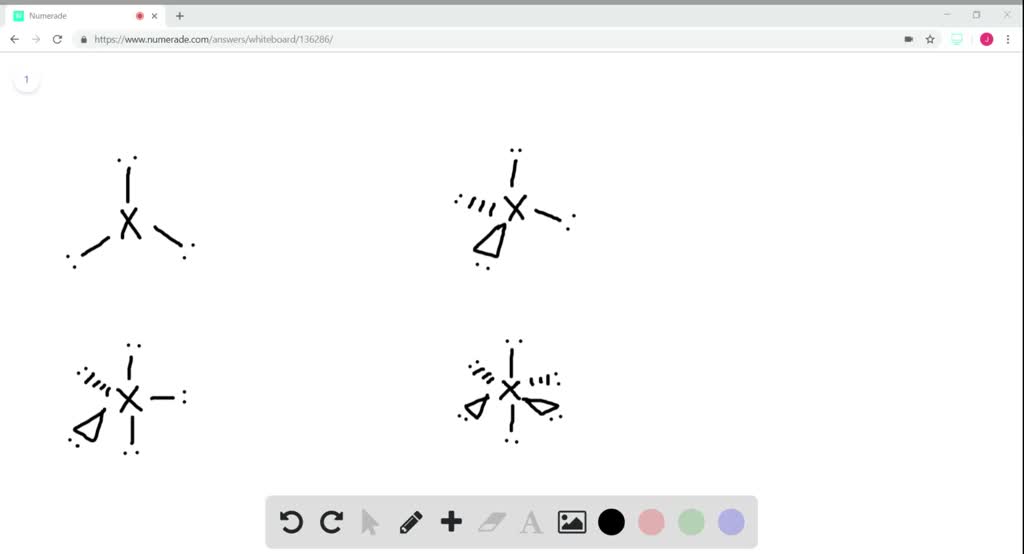
Overview: H2S electron and molecular geometry.Key Points To Consider When drawing The H2S Molecular Geometry.In the tenth B site, B is bonded in a 9-coordinate geometry to six Mg and three B atoms. In the ninth B site, B is bonded in a 9-coordinate geometry to six Mg and three B atoms. In the eighth B site, B is bonded in a 9-coordinate geometry to one Li, five Mg, and three B atoms.

There is one shorter (1.76 Å) and one longer (1.77 Å) B–B bond length. In the seventh B site, B is bonded in a 9-coordinate geometry to six Mg and three B atoms. There is one shorter (1.75 Å) and one longer (1.79 Å) B–B bond length. In the sixth B site, B is bonded in a 9-coordinate geometry to one Li, five Mg, and three B atoms. In the fifth B site, B is bonded in a 9-coordinate geometry to one Li, five Mg, and three B atoms. In the fourth B site, B is bonded in a 9-coordinate geometry to one Li, five Mg, and three B atoms. In the third B site, B is bonded in a 9-coordinate geometry to one Li, five Mg, and three B atoms. In the second B site, B is bonded in a 9-coordinate geometry to six Mg and three B atoms. In the first B site, B is bonded in a 9-coordinate geometry to one Li, five Mg, and three B atoms. There are a spread of Mg–B bond distances ranging from 2.48–2.52 Å. In the fifth Mg site, Mg is bonded to twelve B atoms to form MgB12 cuboctahedra that share an edgeedge with one LiB12 cuboctahedra, edges with eleven MgB12 cuboctahedra, a faceface with one LiB12 cuboctahedra, and faces with seven MgB12 cuboctahedra. There are a spread of Mg–B bond distances ranging from 2.47–2.51 Å. In the fourth Mg site, Mg is bonded to twelve B atoms to form MgB12 cuboctahedra that share an edgeedge with one LiB12 cuboctahedra, edges with eleven MgB12 cuboctahedra, a faceface with one LiB12 cuboctahedra, and faces with seven MgB12 cuboctahedra. There are a spread of Mg–B bond distances ranging from 2.48–2.51 Å. In the third Mg site, Mg is bonded to twelve B atoms to form MgB12 cuboctahedra that share an edgeedge with one LiB12 cuboctahedra, edges with eleven MgB12 cuboctahedra, a faceface with one LiB12 cuboctahedra, and faces with seven MgB12 cuboctahedra.
There are a spread of Mg–B bond distances ranging from 2.49–2.51 Å. In the second Mg site, Mg is bonded to twelve B atoms to form MgB12 cuboctahedra that share edges with three equivalent LiB12 cuboctahedra, edges with nine MgB12 cuboctahedra, and faces with eight MgB12 cuboctahedra. There are a spread of more » Mg–B bond distances ranging from 2.48–2.50 Å. In the first Mg site, Mg is bonded to twelve B atoms to form MgB12 cuboctahedra that share edges with twelve MgB12 cuboctahedra, faces with two equivalent LiB12 cuboctahedra, and faces with six MgB12 cuboctahedra. There are a spread of Li–B bond distances ranging from 2.48–2.50 Å. Li is bonded to twelve B atoms to form LiB12 cuboctahedra that share edges with twelve MgB12 cuboctahedra and faces with eight MgB12 cuboctahedra. LiMg9B20 is hexagonal omega structure-derived structured and crystallizes in the triclinic P-1 space group.


 0 kommentar(er)
0 kommentar(er)
